Allylic aryl benzylic n h h o h o oh nh2 o sh ch3 o oh h2 n oh oh o h o oh2 above 50 7 2 to 3 2 to 3 4 5 9 10 10 10 16 18 18 20 25 25 38 40 41 43 43 45 50.
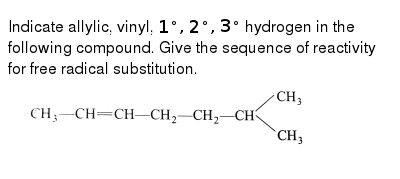
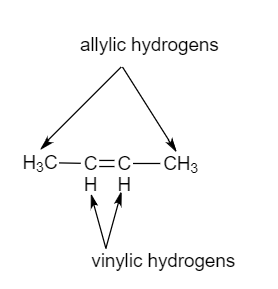
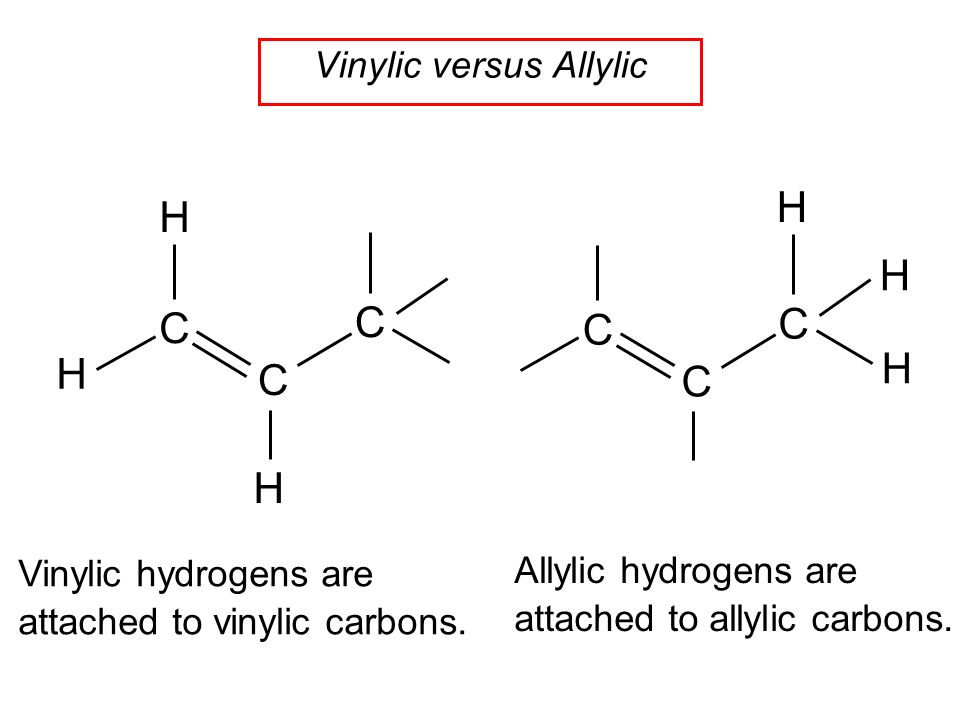
Vinylic vs allylic hydrogen.
So i ve never been able to keep these terms straight and i m having trouble finding a clear cut answer.
This increases the acidity of an allylic proton.
The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms.
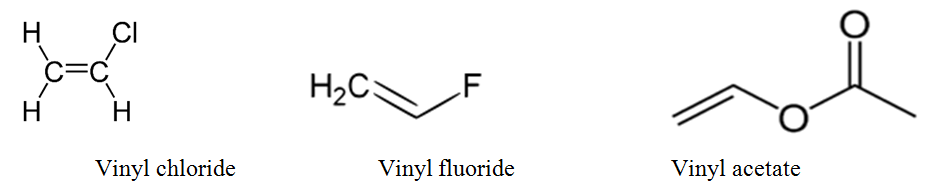
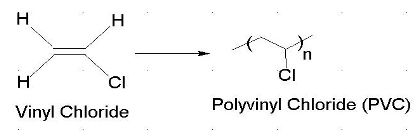
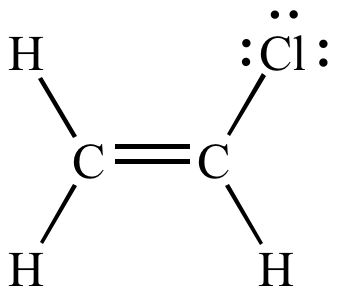
An industrially important example is vinyl chloride precursor to pvc a plastic.
In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom.
Vinyl halides have a pka of around 44 depending on the groups attached to the alkene so yeah not acidic at all.
Professor heath s chemistry channel 9 561 views.
Allyl group holds three carbon atoms and five hydrogen atoms on the other hand vinyl group has two carbon atoms and three hydrogen atoms.
Compound bound to a c bound to a c c so x in c c c x would be an allylic group.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
Allyl groups have three carbon atoms and five hydrogen atoms.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
Here are my best guesses.
The allylic carbon atom is more reactive than normal.
Allylic the conjugate base would be delocalized through the conjugated pi system stabilizing it.
We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739.
Microsoft word functional group pka author.
For the love of physics walter lewin.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.